In physics, the term “broadening” is used to describe the phenomenon where certain features of a system—such as spectral lines or particle distributions—spread or become less sharp. This can occur due to a variety of physical processes, and understanding the different types of broadening is crucial in areas such as quantum mechanics, spectroscopy, and statistical mechanics. Broadening in physics can help explain a wide range of experimental observations and is integral to our understanding of interactions between particles, atoms, and fields.
In this blog, we will explore the concept of broadening in physics, break down its different types, and provide relevant examples to help illustrate how broadening manifests in various physical contexts.
What is Broadening in Physics?
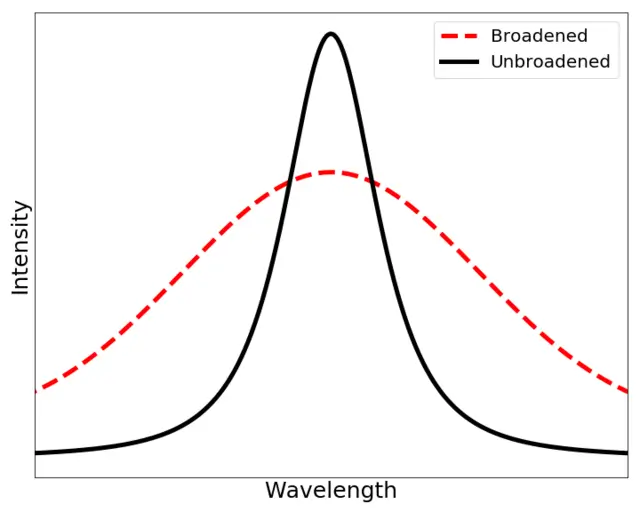
Broadening refers to the spreading or widening of a feature, typically a peak or a line, in a physical system. In most cases, this feature is represented as a sharp point, such as a spectral line or a distribution curve, but broadening causes it to become more spread out or diffuse.
In the context of physics, broadening can occur in different scenarios, but it often arises due to some form of uncertainty, interaction, or disturbance that disturbs the idealized, sharp feature. This change in the shape of the feature may give insight into the underlying physical processes, such as particle interactions, thermal motion, or measurement limitations.
One of the most common examples of broadening is found in spectroscopy, where the sharp lines of light or electromagnetic radiation emitted by atoms or molecules become spread out due to various factors. Broadening can also be seen in statistical mechanics when describing the distribution of energy, or in quantum mechanics when discussing the uncertainty in particle positions and momenta.
Different Types of Broadening in Physics
Broadening can take many forms depending on the underlying cause. Let’s explore the different types of broadening commonly encountered in various branches of physics:
1. Doppler Broadening
Doppler broadening occurs due to the relative motion between the source of radiation and the detector. In atomic or molecular systems, the motion of particles (such as atoms or ions) affects the frequency of the light emitted or absorbed, leading to a spread in the observed spectral lines.
Example: Spectral Lines of Moving Atoms
When atoms or molecules move towards or away from a light source, the frequency of the emitted or absorbed radiation changes due to the Doppler effect. If atoms are moving at different speeds in random directions, this results in a broadening of the spectral line because of the distribution of velocities. The faster the particles are moving, the greater the Doppler shift and, therefore, the broader the observed spectral line.
This effect is often used in astronomy to determine the velocities of distant objects (e.g., stars or galaxies) by observing the broadening of spectral lines.
2. Pressure Broadening (Collision Broadening)
Pressure broadening arises when atoms or molecules collide with one another. These collisions affect the energy levels of the particles, leading to a spread in the frequencies of emitted or absorbed radiation. This is particularly significant in gases under high pressure, where the frequency of the light emitted or absorbed by the atoms or molecules becomes broadened due to these interactions.
Example: Spectral Lines in High-Density Gases
In a gas under high pressure, the frequent collisions between atoms or molecules cause perturbations in the energy levels of the particles, which leads to a broadening of the spectral lines. For example, in a gas of hydrogen atoms at high pressure, the Balmer series of hydrogen lines (visible spectral lines) can broaden due to these collisions. The broader the spectral lines, the higher the pressure and the more frequent the collisions.
3. Natural Broadening (Quantum Mechanical Broadening)
Natural broadening, or sometimes called lifetime broadening, is an intrinsic effect caused by the finite lifetime of excited states in quantum systems. According to the Heisenberg uncertainty principle, the uncertainty in energy is inversely proportional to the uncertainty in the time it takes for a system to relax from an excited state. Thus, a finite lifetime of the excited state leads to an uncertainty in energy, which causes the spectral line to broaden.
Example: Spontaneous Emission of Radiation
When an atom is in an excited state, it will eventually decay to a lower energy state by emitting radiation. The uncertainty in the time taken for this decay leads to a broadening of the emission line. This is known as natural broadening, and it is an inherent property of quantum systems. For example, the emission lines of hydrogen or other atoms are broadened due to the finite lifetime of the excited states.
4. Instrumental Broadening
Instrumental broadening is a result of limitations in the resolution of the measuring instruments, such as spectrometers or detectors. The equipment’s ability to distinguish between closely spaced frequencies or energies determines the sharpness of the spectral lines observed. If the instrument has finite resolution, it can cause the observed lines to appear broader than they actually are.
Example: Spectrometer Resolution
In a spectroscopic experiment, if the spectrometer has limited resolution, it cannot distinguish between very closely spaced energy levels or frequencies. As a result, the spectral lines measured will appear broader than the actual lines emitted by the atom or molecule. This type of broadening is an artifact of the measurement process and can be minimized by using higher-resolution instruments.
5. Thermal Broadening
Thermal broadening occurs due to the motion of particles in a system at finite temperature. At higher temperatures, the atoms or molecules in a gas or solid move with greater velocities, which results in a broadening of spectral lines, similar to Doppler broadening. This effect is particularly important when studying the spectra of gases or plasmas at high temperatures.
Example: Thermal Broadening in Atomic Spectra
At higher temperatures, the atoms in a gas are moving at higher speeds. This motion causes a Doppler shift in the radiation emitted or absorbed by the atoms, leading to a broadening of the spectral lines. The broader the lines, the higher the temperature of the system. This thermal broadening is particularly noticeable in hot stars or laboratory plasmas.
6. Magnetic Field Broadening (Zeeman Broadening)
When an atom or molecule is placed in a magnetic field, the energy levels split due to the interaction between the magnetic moment of the particle and the external field. This splitting leads to a broadening of the spectral lines, known as Zeeman broadening. The amount of broadening depends on the strength of the magnetic field and the properties of the atom or molecule.
Example: Zeeman Effect in Atomic Spectra
In the presence of a magnetic field, spectral lines of atoms (such as hydrogen or sodium) split into multiple components, a phenomenon known as the Zeeman effect. The presence of the magnetic field causes the energy levels of the atoms to split slightly, leading to broadening and splitting of the observed spectral lines. The broader the lines, the stronger the magnetic field.
7. Superposition Broadening (Line Shape Broadening)
Superposition broadening occurs when multiple overlapping processes contribute to the broadening of a spectral line. Each broadening mechanism (Doppler, pressure, natural, etc.) might cause the line to spread, and the combined effect of these mechanisms can lead to a final broadening that is different from any individual contribution. In many cases, this type of broadening can lead to complex line shapes that are difficult to model.
Example: Broadening of Spectral Lines in the Sun’s Atmosphere
In the solar atmosphere, spectral lines from various atoms and ions are broadened by multiple processes: Doppler shifts due to thermal motion, pressure broadening due to the dense solar gas, and instrumental broadening from the spectroscopic measurements. The combination of these factors results in spectral lines with complicated, non-Gaussian shapes.
Conclusion
Broadening is a fundamental concept in many areas of physics, including spectroscopy, quantum mechanics, and statistical mechanics. It refers to the spreading or diffusion of spectral lines, particle distributions, or other physical features due to a variety of factors. Whether due to Doppler shifts, collisions, quantum effects, or limitations of measuring instruments, broadening provides valuable information about the underlying physical processes.
By understanding the different types of broadening and their causes, we can gain deeper insights into the behavior of particles, atoms, and molecules in various physical environments. Broadening is not only a useful tool in experimental physics but also an essential concept for analyzing and interpreting data in fields like astronomy, plasma physics, and quantum theory.




