In the realm of physics, concepts like work function and electron volt play crucial roles in understanding the behaviour of particles, particularly electrons, within materials. These concepts are fundamental to comprehend various phenomena ranging from the photoelectric effect to semiconductor physics. In this article, we delve into the definitions, examples, and significance of work function and electron volt in the realm of physics.
Work Function:
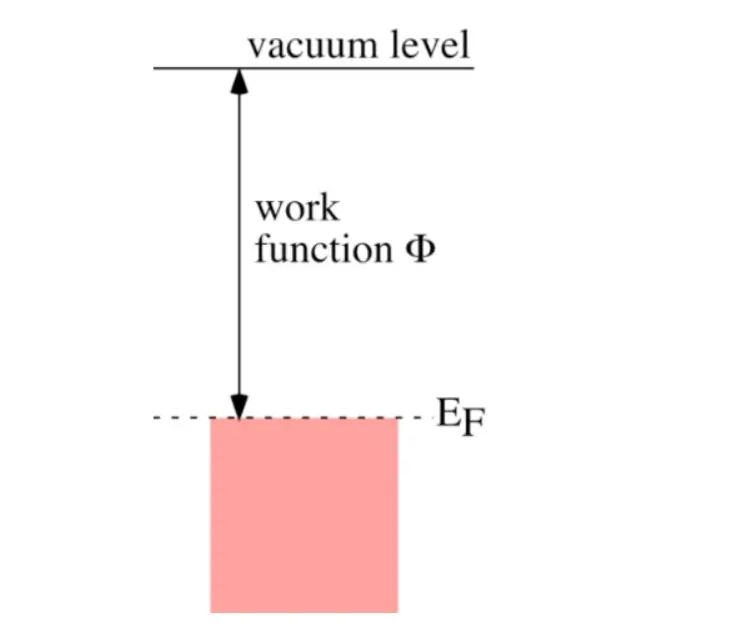
Work function, denoted by the symbol Φ (phi), is a fundamental concept in quantum mechanics and solid-state physics. It represents the minimum amount of energy required to remove an electron from a material to a point just outside its surface.
Mathematically, work function is defined as the difference between the energy of the vacuum level and the Fermi level (the highest occupied energy level) within the material.
Symbolically, the work function (Φ) can be expressed as:
Φ = E vacuum – E Fermi
Where:
- Φ is the work function.
- E vacuum is the energy of the vacuum level.
- E Fermi is the Fermi energy level.
Examples:
- Photoelectric Effect: One of the classic examples illustrating the concept of work function is the photoelectric effect. When light of sufficient energy (at least equal to the work function) strikes the surface of a material, it can eject electrons from the material. The minimum frequency (or maximum wavelength) of light required to cause this effect is directly related to the work function of the material.
- Semiconductor Physics: In semiconductor physics, the work function plays a crucial role in determining the behaviour of electron flow between different materials, such as metal-semiconductor or semiconductor-semiconductor junctions. The difference in work function between materials at a junction influences the direction and magnitude of electron flow, contributing to the operation of devices like diodes and transistors.
- Surface Science: Understanding the work function is essential in surface science and materials engineering, particularly in applications involving electron emission from surfaces, such as field emission microscopy and electron spectroscopy techniques.
Electron Volt (eV):
The electron volt (eV) is a unit of energy commonly used in physics, especially in atomic and particle physics. It is defined as the amount of energy gained (or lost) by an electron when it moves through an electric potential difference of one volt.
Mathematically, one electron volt is equal to the charge of an electron (1.6 × 10^-19 coulombs) multiplied by one volt:
1 eV = 1.6 × 10^-19 J
The electron volt provides a convenient and intuitive way to express energy on the atomic and subatomic scale, where energies are often very small. For example, the energy levels of electrons in atoms and the work function of materials are typically expressed in electron volts rather than joules.
Significance:
Understanding work function and electron volt is crucial in various fields of physics and materials science. They are foundational concepts that underpin our understanding of electron behaviour in materials, from basic principles like the photoelectric effect to complex semiconductor devices. Moreover, the electron volt provides a practical unit for quantifying energy at the atomic and subatomic levels, facilitating calculations and measurements in diverse areas of physics research and technology development.




