In the realm of materials science, the behaviour of substances regarding the flow of electric charge is categorised into three main types: conductors, insulators, and semiconductors. These distinctions are fundamental to various technological applications, ranging from basic circuitry to advanced electronics. To comprehend these concepts fully, it’s crucial to delve into the underlying principles of electron behaviour within materials, particularly concerning valence bands, conduction bands, and bandgap in semiconductors.
Conductors:
Definition: Conductors are materials that permit the flow of electrical current with little resistance. In conductors, electrons are loosely bound to their atoms, allowing them to move freely in response to an electric field.
Characteristics:
- High Electrical Conductivity: Conductors possess a high number of free electrons available for conduction. Metals like copper and aluminium are classic examples of conductors due to their abundance of free electrons.
- Low Resistivity: Conductors exhibit low resistance to the flow of electric current. This low resistance results from the ease with which electrons can move through the material.
- External Influence: The conductivity of a conductor can be influenced by factors such as temperature, impurities, and mechanical stress.
Insulators:
Definition: Insulators are materials that inhibit the flow of electrical current. In insulators, electrons are tightly bound to their atoms, preventing their movement in response to an electric field.
Characteristics:
- High Electrical Resistivity: Insulators have very few free electrons available for conduction, resulting in high resistance to electric current flow.
- Wide Bandgap: Insulators typically have a large energy gap between their valence and conduction bands, making it difficult for electrons to transition from the valence band to the conduction band.
- Dielectric Properties: Insulators are often used as dielectric materials in capacitors and electrical insulation due to their ability to store electrical energy without conducting current.
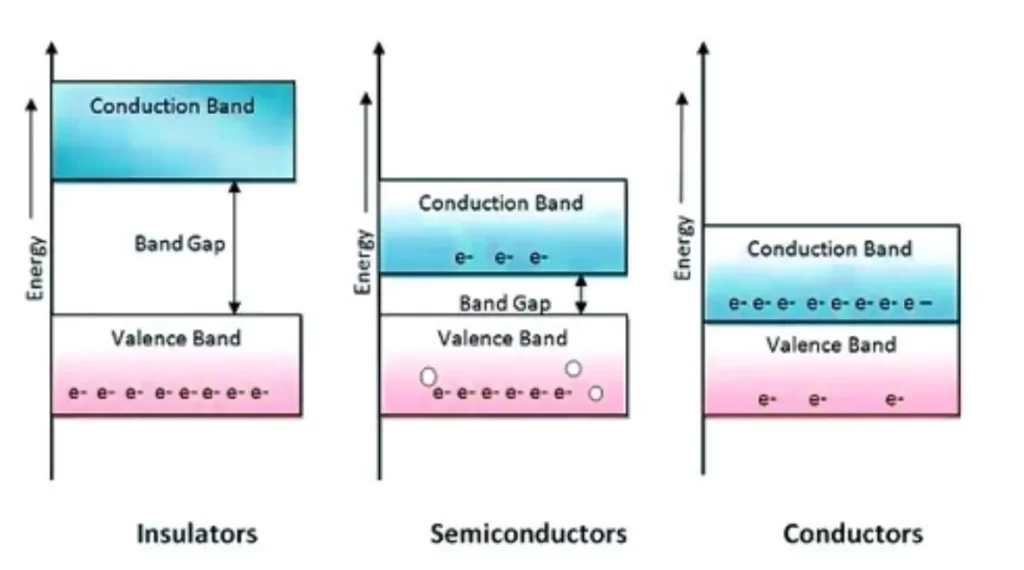
Semiconductors:
Definition: Semiconductors are materials that exhibit electrical conductivity between that of conductors and insulators. Unlike conductors, semiconductors do not readily conduct electricity at room temperature but can do so with the addition of impurities or by applying external factors like temperature.
Characteristics:
- Moderate Electrical Conductivity: Semiconductors have conductivity between that of conductors and insulators. Their conductivity can be increased by doping with specific impurities or by applying an external electric field.
- Band Structure: In semiconductors, there exists a small energy gap, known as the bandgap, between the valence band (where electrons are bound) and the conduction band (where electrons are free to move). The size of this bandgap determines the semiconductor’s conductivity properties.
- Applications: Semiconductors form the basis of modern electronics, including transistors, diodes, and integrated circuits. Their ability to switch between conducting and insulating states makes them invaluable for controlling electrical currents in electronic devices.
Examples of conductors, insulators, and semiconductors:
Conductors:
- Copper: Copper is one of the most widely used conductors due to its high electrical conductivity and availability. It’s commonly utilized in electrical wiring, electronics, and power transmission lines.
- Aluminium: Aluminium is another excellent conductor commonly used in electrical wiring, particularly in power transmission lines and electrical appliances.
- Gold: Gold exhibits exceptional conductivity and corrosion resistance, making it valuable for applications requiring reliable electrical contacts, such as connectors and circuit board traces.
Insulators:
- Rubber: Rubber is an insulating material commonly used for electrical insulation in wires and cables due to its high resistivity and flexibility.
- Glass: Glass is an excellent insulator due to its high electrical resistivity. It’s often utilized in insulating windows, electrical insulators, and as a substrate for electronic components.
- Ceramics: Various ceramic materials, such as porcelain and alumina, are used as insulators in high-temperature and high-voltage applications, including insulators for power lines and electrical equipment.
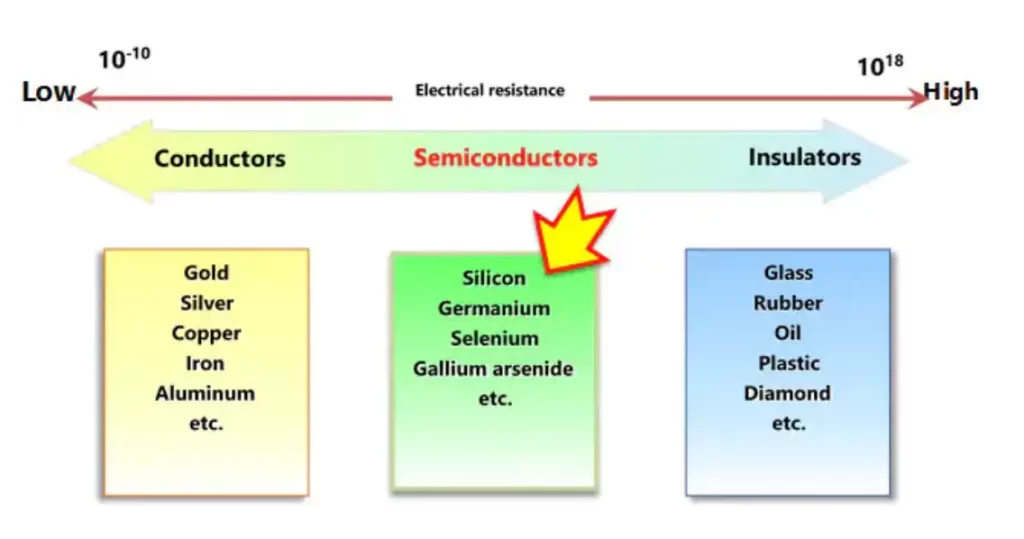
Semiconductors:
- Silicon: Silicon is the most widely used semiconductor material in electronics manufacturing. It’s the foundation of integrated circuits, diodes, transistors, and solar cells due to its abundance and well-understood semiconductor properties.
- Germanium: Germanium was one of the first materials used in semiconductor devices and played a significant role in early transistor technology. While its usage has diminished compared to silicon, it’s still employed in some niche applications.
- Gallium Arsenide (GaAs): Gallium arsenide is a compound semiconductor used in high-frequency electronic devices, such as microwave amplifiers, high-speed digital circuits, and infrared LEDs. Its properties make it suitable for applications where high performance is required.
These examples highlight the diverse range of materials with varying conductivity properties, each suited to specific applications based on their electrical behaviour. Conductors facilitate the flow of electric current, insulators inhibit it, and semiconductors offer a balance between the two, allowing for precise control of electrical conductivity.
Band Theory:
Valence Band: The valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature. Electrons in the valence band are tightly bound to atoms and are involved in forming chemical bonds.
Conduction Band: The conduction band is the range of electron energies above the valence band where electrons are free to move and conduct electricity. For conductors, the conduction band overlaps with the valence band, allowing for easy electron movement. In insulators, there is a large energy gap between the valence and conduction bands, while in semiconductors, this gap is smaller, enabling controlled conductivity.
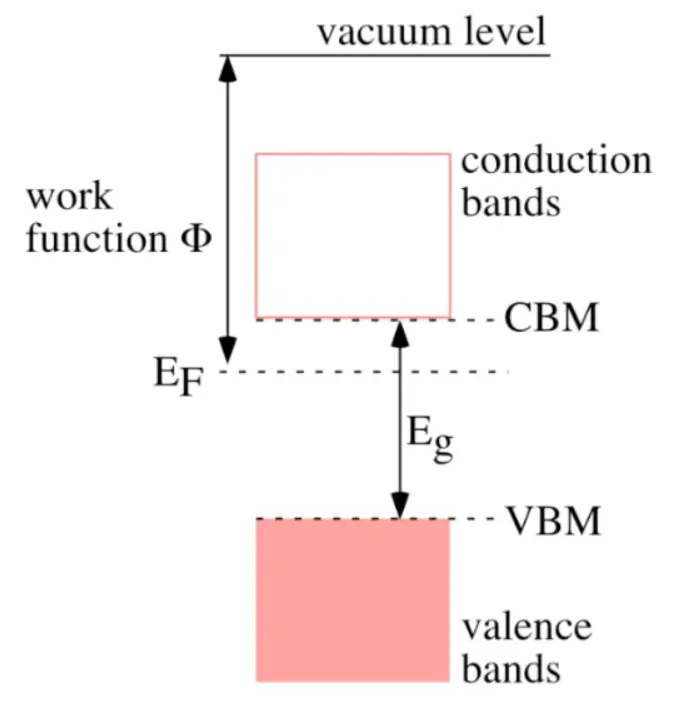
Bandgap: The bandgap is the energy difference between the valence band and the conduction band. In conductors, this gap is essentially non-existent, while in insulators, it is significant. Semiconductors have a relatively small bandgap, allowing them to behave as both conductors and insulators depending on external conditions.
Conclusion:
Understanding the behaviour of materials in terms of conductivity is essential for a wide range of applications, from basic electrical wiring to advanced semiconductor technology. Conductors, insulators, and semiconductors represent the spectrum of electrical behaviour in materials, with their distinctive properties stemming from the arrangement of electrons within their atomic structures. The concepts of valence bands, conduction bands, and bandgap provide a theoretical framework for comprehending the conductivity properties of materials and are foundational to modern electronics and technology.




