Atoms are the fundamental building blocks of matter, forming the basis for everything in the universe. This article delves into the intricate structure of atoms, exploring their components, historical development of atomic theory, and their significance in physics.
Historical Development of Atomic Theory
Ancient Philosophies
The concept of the atom dates back to ancient Greece, where philosophers like Democritus and Leucippus proposed that matter is composed of indivisible units called “atomos.” However, these ideas were largely philosophical and lacked experimental evidence.
Modern Atomic Theory
The modern understanding of the atom began to take shape in the early 19th century with John Dalton’s atomic theory, which proposed that:
- All matter is composed of atoms.
- Atoms of a given element are identical in mass and properties.
- Compounds are formed by combinations of different types of atoms.
- A chemical reaction is a rearrangement of atoms.
Discovering the Electron
In 1897, J.J. Thomson discovered the electron through his experiments with cathode rays. He proposed the “plum pudding” model of the atom, suggesting that electrons were embedded in a positively charged “soup.”
Rutherford's Nuclear Model
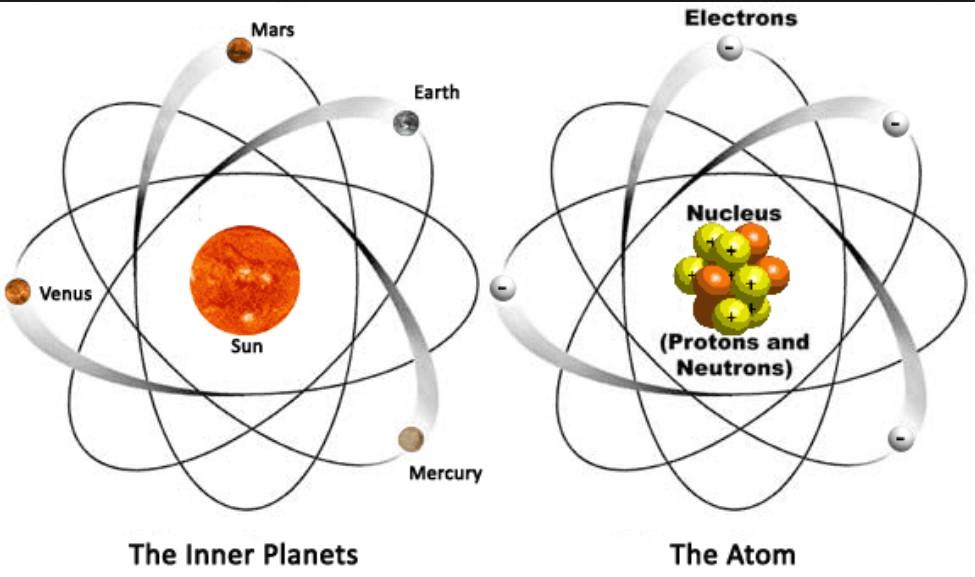
Ernest Rutherford’s gold foil experiment in 1909 led to the discovery of the atomic nucleus. He proposed that an atom consists of a dense, positively charged nucleus surrounded by electrons. This marked the beginning of the nuclear model of the atom.
Bohr Model
Niels Bohr further refined the atomic model in 1913 by introducing quantised electron orbits. He suggested that electrons move in specific orbits around the nucleus, with each orbit corresponding to a fixed energy level.
Quantum Mechanical Model
The quantum mechanical model, developed in the 1920s and 1930s by scientists like Schrödinger, Heisenberg, and Dirac, describes electrons as wave functions rather than particles. This model incorporates the principles of quantum mechanics and provides a probabilistic approach to the behaviour of electrons.
Structure of the Atom
An atom is composed of three primary subatomic particles: protons, neutrons, and electrons. The arrangement and interactions of these particles define the atom’s structure.
The Nucleus
The nucleus is the atom’s dense core, consisting of protons and neutrons.
- Protons: Protons are positively charged particles with a relative mass of 1 atomic mass unit (amu). The number of protons, also known as the atomic number, defines the element.
- Neutrons: Neutrons are neutral particles with a mass slightly greater than that of protons. The number of neutrons can vary among atoms of the same element, leading to different isotopes.
The nucleus is held together by the strong nuclear force, which counteracts the electrostatic repulsion between the positively charged protons.
Electrons and Electron Cloud
Electrons are negatively charged particles that orbit the nucleus. Unlike protons and neutrons, electrons have a negligible mass (approximately 1/1836 of a proton’s mass).
- Electron Shells and Orbitals: Electrons occupy energy levels or shells around the nucleus. Each shell can hold a specific number of electrons, determined by the formula 2n2, where 𝑛 is the principal quantum number. Within these shells, electrons occupy orbitals, which are regions of space where electrons are likely to be found.
- Quantum Mechanics and Wave functions: The behaviour of electrons is described by quantum mechanics. Electrons exist as wave functions, representing the probability distribution of an electron’s position. The Heisenberg Uncertainty Principle states that it is impossible to precisely determine both the position and momentum of an electron simultaneously.
Atomic Models and Their Evolution
The Bohr Model
Bohr’s model introduced the concept of quantised energy levels. Electrons orbit the nucleus in fixed paths or shells, and they can jump between these levels by absorbing or emitting energy in the form of photons.
The Quantum Mechanical Model
The quantum mechanical model, also known as the electron cloud model, provides a more accurate representation of atomic structure. It uses complex mathematical functions to describe the probability of finding an electron in a particular region around the nucleus. This model accounts for the wave-particle duality of electrons and incorporates principles such as:
- Pauli Exclusion Principle: No two electrons can have the same set of quantum numbers, ensuring that each electron in an atom has a unique state.
- Hund’s Rule: Electrons will occupy degenerate orbitals singly before pairing up to minimise electron-electron repulsion.
Isotopes and Atomic Mass
Isotopes
Isotopes are atoms of the same element that have different numbers of neutrons. While they share the same number of protons, their mass numbers differ. For example, carbon-12 and carbon-14 are isotopes of carbon, with 6 and 8 neutrons respectively.
Atomic Mass
The atomic mass of an element is the weighted average of the masses of its isotopes, taking into account their relative abundance. It is usually expressed in atomic mass units (amu), where 1 amu is defined as one-twelfth the mass of a carbon-12 atom.
The Periodic Table and Atomic Structure
The periodic table organises elements based on their atomic number and electron configuration. Elements in the same group (column) have similar chemical properties due to their similar valence electron configurations.
- Periods: Horizontal rows in the periodic table, indicating the number of electron shells.
- Groups: Vertical columns, with elements having the same number of electrons in their outermost shell.
Periodic Trends
- Atomic Radius: Generally decreases across a period and increases down a group.
- Ionisation Energy: The energy required to remove an electron from an atom. It increases across a period and decreases down a group.
- Electronegativity: A measure of an atom’s ability to attract electrons in a chemical bond. It increases across a period and decreases down a group.
Applications of Atomic Theory
Chemistry and Bonding
Understanding atomic structure is crucial for explaining chemical bonding. Atoms bond to achieve stable electron configurations, leading to the formation of molecules and compounds.
- Ionic Bonds: Formed by the transfer of electrons from one atom to another, resulting in positively and negatively charged ions.
- Covalent Bonds: Involve the sharing of electrons between atoms to achieve stability.
- Metallic Bonds: Involve a “sea” of delocalised electrons around positively charged metal ions, giving metals their characteristic properties.
Nuclear Physics
The study of atomic nuclei leads to applications in nuclear energy and medicine. Nuclear reactions, such as fission and fusion, release enormous amounts of energy, while radioactive isotopes are used in medical imaging and treatment.
Quantum Mechanics and Technology
Advancements in quantum mechanics have paved the way for modern technologies such as semiconductors, lasers, and quantum computing. These technologies rely on the principles of electron behaviour at the atomic level.
Conclusion
Atoms and their intricate structures form the foundation of our understanding of the physical world. From ancient philosophical ideas to the sophisticated quantum mechanical models, the study of atomic structure has profoundly impacted science and technology. As we continue to explore the subatomic realm, our knowledge of atoms will undoubtedly lead to new discoveries and innovations.




