Titanium dioxide (TiO2) is one of the most widely studied materials due to its remarkable properties such as high surface area, optical transparency, and photocatalytic activity. These properties make TiO2 an ideal candidate for a variety of applications, including photocatalysis, solar cells, sensors, and thin-film coatings. Among the numerous techniques for synthesizing TiO2 thin films, the sol-gel method combined with spin coating has become one of the most popular and versatile approaches. This blog explores the sol-gel and spin coating methods for synthesizing TiO2 thin films, highlighting their principles, processes, advantages, and challenges in a research context.
1. Introduction to TiO2 Thin Films
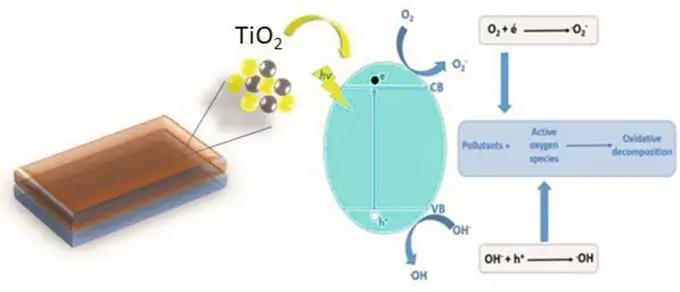
TiO2 thin films are crucial in several advanced technologies due to their exceptional electronic, optical, and photocatalytic properties. TiO2 can exist in three polymorphic forms: anatase, rutile, and brookite. Among these, anatase is particularly favoured for applications in photocatalysis and solar cells due to its higher surface reactivity. The synthesis of high-quality TiO2 thin films is essential to ensure these properties are effectively harnessed for specific applications.
2. Sol-Gel Method for TiO2 Thin Film Synthesis
The sol-gel method is a chemical process that allows the formation of solid materials from small molecules. It is based on the conversion of a solution (sol) into a gel-like network that can be used to form thin films. The sol-gel process is widely used for the deposition of oxide films like TiO2 due to its simplicity, cost-effectiveness, and ability to produce high-purity, homogeneous films.
2.1. Sol-Gel Process Overview
The sol-gel process for TiO2 thin films typically involves several key steps:
- Preparation of Solution (Sol): Titanium tetrachloride (TiCl4), titanium isopropoxide (TIPT), or titanium butoxide (TBT) are commonly used as titanium precursors in sol-gel synthesis. These precursors are dissolved in a solvent (usually alcohol or water), and the sol-gel process begins with the hydrolysis and condensation of the titanium alkoxide precursor.
- Hydrolysis and Condensation: During hydrolysis, water is added to the solution, and the titanium precursor reacts with water molecules to form titanium hydroxide groups. Condensation follows, leading to the formation of a network structure that eventually results in TiO2.
- Formation of Gel: The gelation process begins as the sol transitions into a gel-like state, where the titanium-based sol particles aggregate into a network. The gel may be dried at this point to remove excess solvents.
- Heat Treatment (Calcination): To achieve the desired crystallinity, the gel is heated to high temperatures, typically between 300°C and 500°C, in a furnace. This process ensures that the TiO2 films transition from an amorphous to a crystalline phase (typically anatase), which enhances their optical and photocatalytic properties.
2.2. Advantages of Sol-Gel Method
- Low Cost: The sol-gel method is a low-cost synthesis technique as it uses inexpensive raw materials and simple equipment.
- Control over Stoichiometry: The sol-gel process allows precise control over the composition and structure of the TiO2 film.
- Homogeneity: The sol-gel method produces highly uniform films with excellent adhesion to substrates.
- Low Temperature Processing: The process can be carried out at relatively low temperatures, which is important for applications where high-temperature processes may not be suitable.
2.3. Challenges in Sol-Gel Method
- Long Processing Time: The gelation and calcination steps can be time-consuming.
- Film Thickness Control: It can be challenging to control the thickness and uniformity of the films, especially for thicker films.
- Cracking and Shrinkage: The drying process can cause cracks and shrinkage in the films, particularly when they are deposited on flexible or temperature-sensitive substrates.
3. Spin Coating for Thin Film Deposition
Spin coating is a widely used method for depositing thin films onto substrates, particularly for organic and inorganic materials such as TiO2. This method involves applying a small amount of the sol-gel solution to a flat substrate and then spinning the substrate at high speeds to spread the solution evenly across its surface.
3.1. Spin Coating Process Overview
The spin coating process for TiO2 thin films typically involves the following steps:
- Substrate Preparation: The substrate, usually a silicon wafer or glass slide, is cleaned thoroughly to remove any organic contamination or dust. A common cleaning procedure involves using solvents like acetone and isopropyl alcohol, followed by drying.
- Deposition of Sol-Gel Solution: A few drops of the TiO2 sol (prepared by the sol-gel process) are deposited onto the center of the spinning substrate.
- Spinning the Substrate: The substrate is rapidly spun at a high speed (typically 1000 to 5000 rpm) to spread the sol-gel solution evenly across the surface. The centrifugal force causes the solution to spread outward, forming a thin layer of TiO2.
- Drying: After spinning, the thin film is dried at room temperature or in an oven to evaporate the solvent and leave behind a uniform TiO2 film.
- Annealing or Calcination: After drying, the film is typically annealed in a furnace to remove organic residues and to crystallize the TiO2 into the desired phase (usually anatase).
3.2. Advantages of Spin Coating Method
- Uniform Films: Spin coating can produce thin, uniform films with precise control over thickness.
- Scalability: The method is easily scalable for large-area substrates.
- Simple Equipment: The equipment needed for spin coating is relatively simple and inexpensive, making it ideal for research laboratories.
- Reproducibility: Spin coating allows for high reproducibility of thin-film deposition, ensuring consistency between samples.
3.3. Challenges in Spin Coating Method
- Limited to Flat Substrates: Spin coating is typically used for flat, rigid substrates, making it unsuitable for complex geometries.
- Material Waste: Spin coating often results in significant material waste, as excess sol-gel solution is spun off the substrate.
- Thickness Control: Achieving exact control over film thickness can be difficult, as it depends on several factors, including spin speed, sol concentration, and solvent evaporation rate.
4. Combining Sol-Gel and Spin Coating for TiO2 Thin Films
The combination of the sol-gel method and spin coating provides a powerful approach for fabricating TiO2 thin films with tailored properties. The sol-gel method offers precise control over the chemical composition of the TiO2 precursor, while spin coating ensures a uniform, smooth film deposition.
4.1. Process Steps for Sol-Gel Spin Coating of TiO2 Thin Films:
- Preparation of Sol-Gel Solution: TiO2 precursors (e.g., Ti(IV) isopropoxide) are mixed with solvents such as ethanol or acetylacetone, and the solution is hydrolyzed using an alcohol and water mixture.
- Substrate Cleaning: Substrates (e.g., glass or silicon) are cleaned using solvents to remove organic contamination, ensuring strong adhesion of the TiO2 films.
- Spin Coating: The sol-gel solution is applied to the substrate and spun at high speeds to create a thin, uniform TiO2 layer.
- Drying and Annealing: The film is dried to remove solvents, followed by annealing at temperatures ranging from 400°C to 500°C to crystallize the TiO2 phase and improve film quality.
5. Characterization Techniques for TiO2 Thin Films
After the deposition of TiO2 thin films, several characterization techniques are employed to analyze the structural, optical, and morphological properties of the films:
- X-ray Diffraction (XRD): Used to determine the crystallinity and phase of TiO2 (anatase, rutile, or mixed phases).
- Scanning Electron Microscopy (SEM): Provides high-resolution images of the film’s surface morphology.
- Atomic Force Microscopy (AFM): Used to measure the surface roughness and topography of TiO2 films.
- UV-Vis Spectroscopy: Used to study the optical properties, such as the band gap of TiO2 films.
- Photocatalytic Testing: To evaluate the photocatalytic efficiency of TiO2 films, especially in environmental and energy applications.
6. Applications of TiO2 Thin Films
TiO2 thin films synthesized by the sol-gel and spin coating methods have a wide range of applications, including:
- Photocatalysis: TiO2 is extensively used in environmental cleanup, self-cleaning coatings, and water splitting.
- Solar Cells: TiO2 films are used in dye-sensitized solar cells (DSSCs) as the photoanode.
- Optical Coatings: TiO2 is used in anti-reflective coatings and optical filters due to its high refractive index.
- Sensors: TiO2 films are used in gas sensors and biosensors due to their sensitivity to UV light and gases like nitrogen dioxide.
Conclusion
The sol-gel and spin coating methods offer an efficient, cost-effective approach to synthesizing high-quality TiO2 thin films. The sol-gel process allows for precise control over the chemical composition of the precursor solution, while spin coating ensures uniform deposition on substrates. These methods are particularly useful in research settings where the production of thin, homogeneous films with controlled properties is required. Despite some challenges, such as film cracking and thickness control, these methods are widely applied for the fabrication of TiO2 films with excellent photocatalytic and optical properties, making them essential in various technological fields. Researchers continue to explore improvements in these methods to enhance the performance and applicability of TiO2-based thin films in emerging technologies.




