Materials play a fundamental role in the development of technology, manufacturing, and everyday life. The properties of materials can significantly vary based on their internal structure, which influences their strength, conductivity, optical properties, and even their resistance to wear and corrosion. Two of the most important categories in materials science are single crystalline and polycrystalline materials. These materials are distinguished primarily by the way their atoms are arranged, which impacts their properties and applications.
In this blog, we will explore the differences between single crystalline and polycrystalline materials, their characteristics, examples, and how they are used in various industries.
What is a Single Crystalline Material?
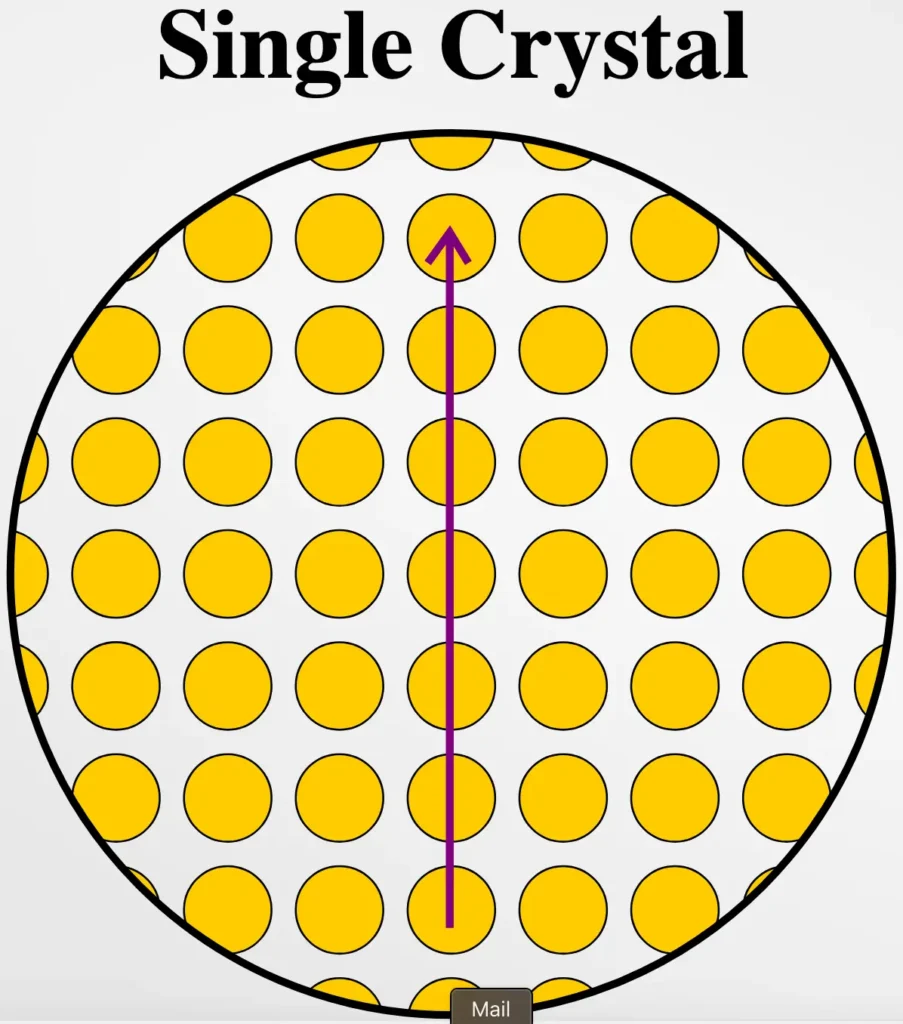
A single crystalline material (also known as a single crystal) is a material that consists of a continuous, uninterrupted lattice of atoms. This means that the atoms are arranged in a repetitive and orderly manner across the entire sample. There are no grain boundaries or defects within the crystal. The structure is uniform throughout the material, which gives it unique properties.
Key Features of Single Crystalline Materials:
- Uniform Atomic Structure: The atoms are arranged in a repeating pattern in all directions, making it a perfect lattice.
- Superior Strength and Durability: Because of the lack of grain boundaries, single crystals often have higher mechanical strength in specific directions.
- Electrical and Thermal Conductivity: Single crystalline materials can have very high electrical and thermal conductivity along certain crystallographic directions.
- Optical Properties: Many single crystals exhibit unique optical properties due to their highly ordered atomic structure.
Examples of Single Crystalline Materials:
- Silicon: Single crystalline silicon is used extensively in semiconductor devices, including computer processors, solar cells, and sensors. Its high electrical conductivity, combined with its well-understood properties, makes it the backbone of modern electronics.
- Diamond: As a natural single crystal, diamond is known for its extreme hardness and excellent thermal conductivity. It is used in cutting tools, high-performance heat sinks, and even in some quantum computing applications.
- Sapphire: Single crystalline sapphire (aluminium oxide, Al₂O₃) is used in a wide range of applications such as LED production, high-strength windows, and optical components. Its high thermal conductivity and optical clarity make it ideal for use in harsh environments.
- Gallium Arsenide (GaAs): This is a semiconductor material used in high-speed devices like transistors, microwave circuits, and laser diodes.
What is a Polycrystalline Material?
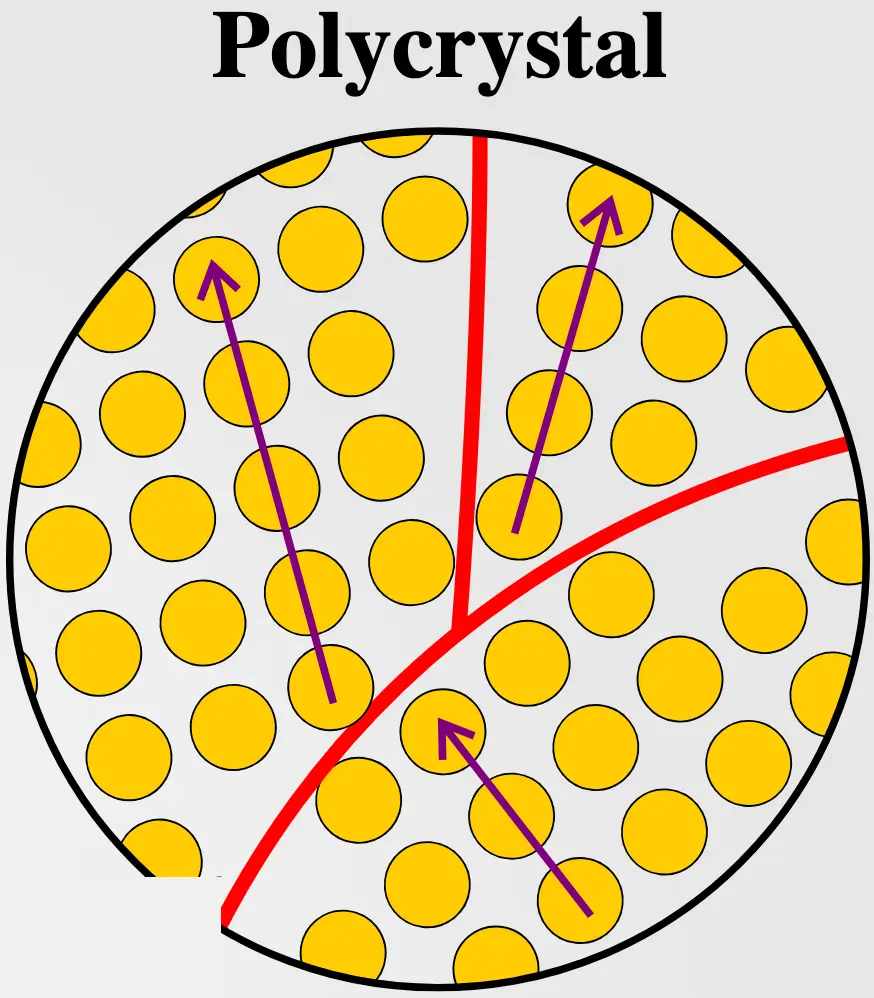
Polycrystalline materials are composed of many small crystals or grains, each with its own orientation. These individual crystals are known as grains, and while they are aligned in different directions, the boundaries between them are typically random. Grain boundaries can have a significant effect on the material’s mechanical, electrical, and optical properties.
Key Features of Polycrystalline Materials:
- Grain Boundaries: Unlike single crystals, polycrystals have boundaries between their grains, which can impede the movement of dislocations, making polycrystals generally stronger in compression.
- Isotropic Properties: The presence of many grains gives polycrystalline materials more uniform properties in all directions, as opposed to the directional properties of single crystals.
- Electrical and Thermal Conductivity: In polycrystalline materials, the grain boundaries can scatter electrons or phonons, leading to lower electrical and thermal conductivity compared to single crystals.
- Toughness: The grain boundaries in polycrystalline materials can improve toughness, making these materials less likely to fracture under stress.
Examples of Polycrystalline Materials:
- Metals: Most metals, such as steel, aluminium, and copper, are polycrystalline in nature. In fact, these materials often undergo processes like forging or casting, which cause them to develop a polycrystalline structure. These metals are used in construction, machinery, and electronics.
- Ceramics: Porcelain and brick are examples of polycrystalline ceramics. The grains in these materials contribute to their hardness and ability to withstand high temperatures.
- Solar Panels: Polycrystalline silicon is used in solar cells. While not as efficient as single-crystal silicon, polycrystalline silicon is more cost-effective and is widely used for commercial solar power generation.
- Superconducting Materials: Certain high-temperature superconductors, such as YBCO (Yttrium Barium Copper Oxide), are polycrystalline and exhibit superconductivity when cooled below a certain temperature.
Differences Between Single Crystalline and Polycrystalline Materials
Feature | Single Crystalline Materials | Polycrystalline Materials |
|---|---|---|
Atomic Structure | Continuous and uniform lattice | Many small grains with different orientations |
Mechanical Properties | Higher strength in specific directions | Higher toughness and better strength in compression |
Electrical Conductivity | High conductivity in specific directions | Lower conductivity due to grain boundaries |
Thermal Conductivity | High conductivity along certain directions | Lower conductivity due to grain boundaries |
Manufacturing Complexity | More difficult to manufacture | Easier and more cost-effective to produce |
Applications | Semiconductors, aerospace, optics | Construction, energy, bulk materials |
Advantages and Disadvantages of Single Crystalline Materials
Advantages:
- High Performance: Single crystalline materials, especially semiconductors like silicon, provide superior performance in applications requiring precise electrical properties.
- Strength in Specific Directions: Due to the uninterrupted atomic structure, these materials can exhibit exceptional mechanical strength in particular orientations.
- Thermal and Electrical Conductivity: These materials often outperform polycrystals in both electrical and thermal conductivity.
Disadvantages:
- Cost and Manufacturing Complexity: Producing single crystalline materials can be costly and technologically challenging, requiring advanced techniques like the Czochralski process or chemical vapor deposition (CVD).
- Brittleness: While strong, single crystals can be more brittle under certain conditions, as the absence of grain boundaries leaves little room for plastic deformation.
Advantages and Disadvantages of Polycrystalline Materials
Advantages:
- Cost-Effective: Polycrystalline materials are generally cheaper and easier to produce, especially for bulk applications.
- Toughness: The grain boundaries in polycrystals contribute to their resistance to cracking, making them more durable under stress.
- Versatility: Polycrystalline materials can be engineered for a variety of applications, from everyday building materials to high-tech ceramics and semiconductors.
Disadvantages:
- Lower Conductivity: The grain boundaries hinder the flow of electricity and heat, which reduces the efficiency of these materials in some high-performance applications.
- Directional Properties: While polycrystals have more uniform properties overall, they may not have the exceptional directional properties of single crystalline materials.
Conclusion
Both single crystalline and polycrystalline materials are essential to modern technology and manufacturing, each with its own set of advantages and limitations. Single crystalline materials are invaluable in fields that require high precision, electrical conductivity, or optical clarity, while polycrystalline materials are crucial in applications that prioritize strength, toughness, and cost-effectiveness.
The decision to use single crystalline or polycrystalline materials depends largely on the specific requirements of the application—whether it’s the high efficiency needed for electronics and semiconductors, or the durability required in construction and bulk materials. Understanding these materials and their properties is key to optimizing their use in modern industry.




