Accuracy, Precision and Uncertainty in Measurement:
Physics is a science based on observations and experiments. Observations of various physical quantities are made during an experiment.
For example, during the atmospheric study we measure atmospheric pressure, wind velocity, humidity, etc. All the measurements may be accurate, meaning that the measured values are the same as the true values.
Accuracy is how close a measurement is to the actual value of that quantity. These measurements may be precise, meaning that multiple measurements give nearly identical values (i.e., reproducible results). In actual measurements, an observation may be both accurate and precise or neither accurate nor precise. The goal of the observer should be to get accurate as well as precise measurements.
Possible uncertainties in an observation may arise due to following reasons:
1) Quality of instrument used.
2) Skill of the person doing the experiment.
3) The method used for measurement.
4) External or internal factors affecting the result of the experiment.
Errors in Measurements:
Faulty measurements of physical quantity can lead to errors. The errors are broadly divided into the following two categories :
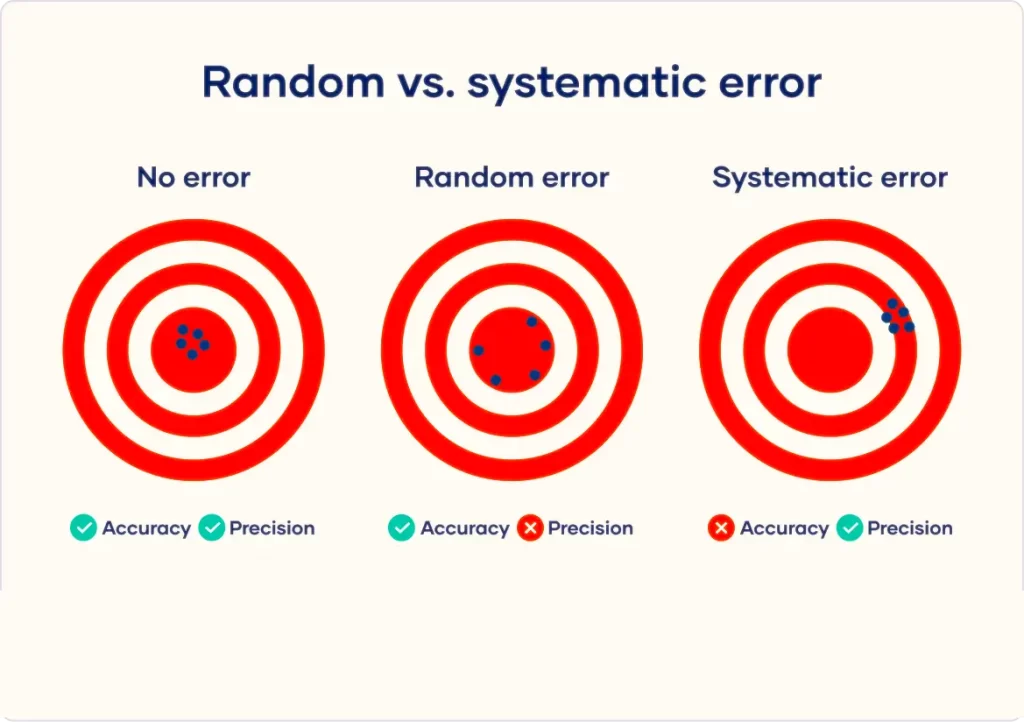
a) Systematic errors :
Systematic errors are errors that are not determined by chance but are introduced by an inaccuracy (involving either the observation or measurement process) inherent to the system. Sources of systematic error may be due to imperfect calibration of the instrument, and sometimes imperfect method of observation.
Each of these errors tends to be in one direction, either positive or negative. The sources of systematic errors are as follows:
(i) Instrumental error: This type of error arises due to defective calibration of an instrument, for example an incorrect zeroing of an instrument will lead to such kind of error (‘zero’ of a thermometer not graduated at proper place, the pointer of weighting balance in the laboratory already indicating some value instead of showing zero when no load is kept on it, an ammeter showing a current of 0.5 amp even when not connected in circuit, etc).
(ii) Error due to imperfection in experimental technique: This is an error due to defective setting of an instrument.
For example the measured volume of a liquid in a graduated tube will be inaccurate if the tube is not held vertical.
(iii)Personal error: Such errors are introduced due to fault of the observer. Bias of the observer, carelessness in taking observations etc. could result in such errors.
For example, while measuring the length of an object with a ruler, it is necessary to look at the ruler from directly above. If the observer looks at it from an angle, the measured length will be wrong due to parallax. Systematic errors can be minimised by using correct instrument, following proper experimental procedure and removing personal error.
b) Random errors:
These are the errors which are introduced even after following all the procedures to minimise systematic errors. These type of errors may be positive or negative. These errors can not be eliminated completely but we can minimise them by repeated observations and then taking their mean (average). Random errors occur due to variation in conditions in which experiment is performed.
For example, the temperature may change during the course of an experiment, pressure of any gas used in the experiment may change, or the voltage of the power supply may change randomly, etc.
Significant Figures:
In the previous sections, we have studied various types of errors, their origins and the ways to minimise them. Our accuracy is limited to the least count of the instrument used during the measurement. Least count is the smallest measurement that can be made using the given instrument.
For example with the usual metre scale, one can measure 0.1 cm as the least value. Hence its least count is 0.1cm.
Suppose we measure the length of a metal rod using a metre scale of least count 0.1cm. The measurement is done three times and the readings are 15.4, 15.4, and 15.5 cm. The most probable length which is the arithmetic mean as per our earlier discussion is 15.43. Out of this we are certain about the digits 1 and 5 but are not certain about the last 2 digits because of the least count limitation.
The number of digits in a measurement about which we are certain, plus one additional digit, the first one about which we are not certain is known as significant figures or significant digits.
Thus in above example, we have 3 significant digits 1, 5 and 4. The larger the number of significant figures obtained in a measurement, the greater is the accuracy of the measurement. If one uses the instrument of smaller least count, the number of significant digits increases.
Rules for determining significant figures
- All the nonzero digits are significant, for example if the volume of an object is 178.43 cm3, there are five significant digits which are 1,7,8,4 and 3.
- All the zeros between two nonzero digits are significant, eg., m = 165.02 g has 5 significant digits.
- If the number is less than 1, the zero/zeroes on the right of the decimal point and to the left of the first nonzero digit are not significant e.g. in 0.001405, the underlined zeros are not significant. Thus the above number has four significant digits.
- The zeros on the right hand side of the last nonzero number are significant (but for this, the number must be written with a decimal point), e.g. 1.500 or 0.01500 have both 4 significant figures each.
On the contrary, if a measurement yields length L given as
L = 125 m = 12500 cm = 125000 mm, it has only three significant digits.
To avoid the ambiguities in determining the number of significant figures, it is necessary to report every measurement in scientific notation (i.e., in powers of 10) i.e., by using the concept of order of magnitude.
The magnitude of any physical quantity can be expressed as A×10n where ‘A’ is a number such that 0.5≤A<5 and ‘n’ is an integer called the order of magnitude.
(i) radius of Earth = 6400 km = 0.64×107m
The order of magnitude is 7 and the number of significant figures are 2.
(ii) Magnitude of the charge on electron e = 1.6×10-19 C
Here the order of magnitude is -19 and the number of significant digits are 2.




