Gases play a crucial role in our daily lives, and understanding their behaviour is essential for various scientific and industrial applications. The concepts of ideal gases and real gases serve as fundamental models to explain the behaviour of gases under different conditions. In this comprehensive article, we will delve into the characteristics, equations, and deviations associated with ideal and real gases.
Ideal Gases:
Characteristics of Ideal Gases:
- Molecular Nature:
Ideal gases are conceptualised as having point-like particles with no volume, mass, or forces of attraction between them. These assumptions simplify the mathematical description of gas behaviour.
- Collisions:
Collisions between ideal gas particles are perfectly elastic, meaning there is no loss of kinetic energy during collisions.
- Negligible Volume:
The volume occupied by the gas particles themselves is considered negligible compared to the total volume of the gas.
- Temperature and Kinetic Energy:
The temperature of an ideal gas is directly proportional to the average kinetic energy of its particles. This relationship is expressed by the equation PV=nRT, known as the ideal gas law, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature.
Ideal Gas Law:
The ideal gas law is a fundamental equation that relates the pressure, volume, and temperature of an ideal gas. It is expressed as:
PV=nRT
where:
P is the pressure of the gas,
V is the volume it occupies,
n is the number of moles of the gas,
R is the ideal gas constant (approximately 8.314 J/(mol·K)),
T is the absolute temperature.
Applications of Ideal Gases:
- Gas Stoichiometry:
Ideal gas law is used to calculate the amount of reactants and products in chemical reactions involving gases.
- Gas Behaviour Predictions:
Ideal gas behaviour is a good approximation at moderate temperatures and low pressures, making it useful in various applications, such as in the design of gas storage and distribution systems.
Real Gases:
Deviations from Ideal Behaviour:
Real gases deviate from ideal behaviour under certain conditions, typically at high pressures and low temperatures. These deviations are attributed to factors not considered in the ideal gas model:
- Volume of Gas Particles:
Real gas particles have a finite volume, which becomes significant at high pressures when the particles are close together.
- Intermolecular Forces:
Real gases experience attractive and repulsive forces between particles. At low temperatures, attractive forces dominate, leading to deviations from ideal behaviour.
- Molecular Size:
The size of real gas molecules becomes significant under high-pressure conditions, causing deviations from the ideal gas law.
Van der Waals Equation:
To account for real gas behaviour, Johannes D. van der Waals introduced an equation that incorporates corrections for particle volume and intermolecular forces:
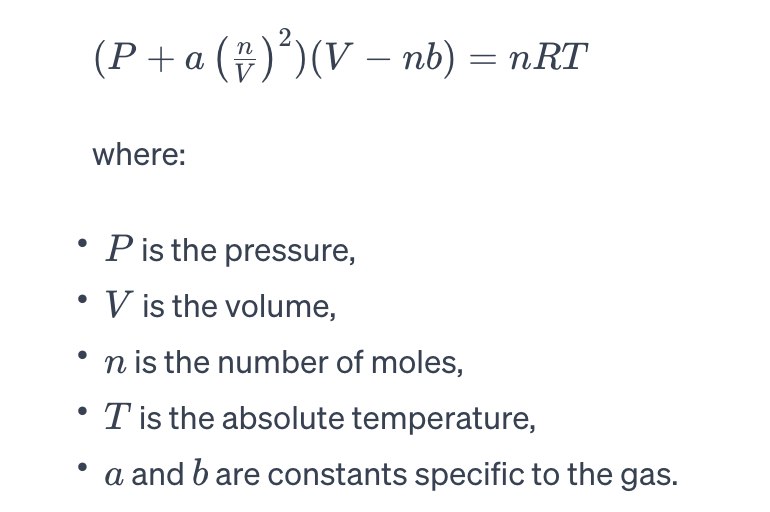
Critical Point:
Every real gas has a set of unique conditions known as its critical point. At this point, the gas cannot be liquefied, regardless of the pressure applied. The critical temperature and critical pressure are essential parameters in understanding real gas behavior.
Applications of Real Gas Concepts:
- Liquefaction of Gases:
Real gas behaviour is crucial in understanding and optimising conditions for liquefying gases.
- High-Pressure Systems:
In applications involving high pressures, understanding real gas behaviour becomes vital for accurate predictions and safe design.
Conclusion:
Ideal gases and real gases serve as essential models for understanding the behaviour of gases in different scenarios. While ideal gases provide a simplified framework for many practical situations, real gases account for the complexities arising from molecular interactions and finite particle volumes. The ideal gas law and the Van der Waals equation offer valuable tools for calculations in diverse fields, from chemistry to engineering, providing a bridge between theoretical concepts and real-world applications.
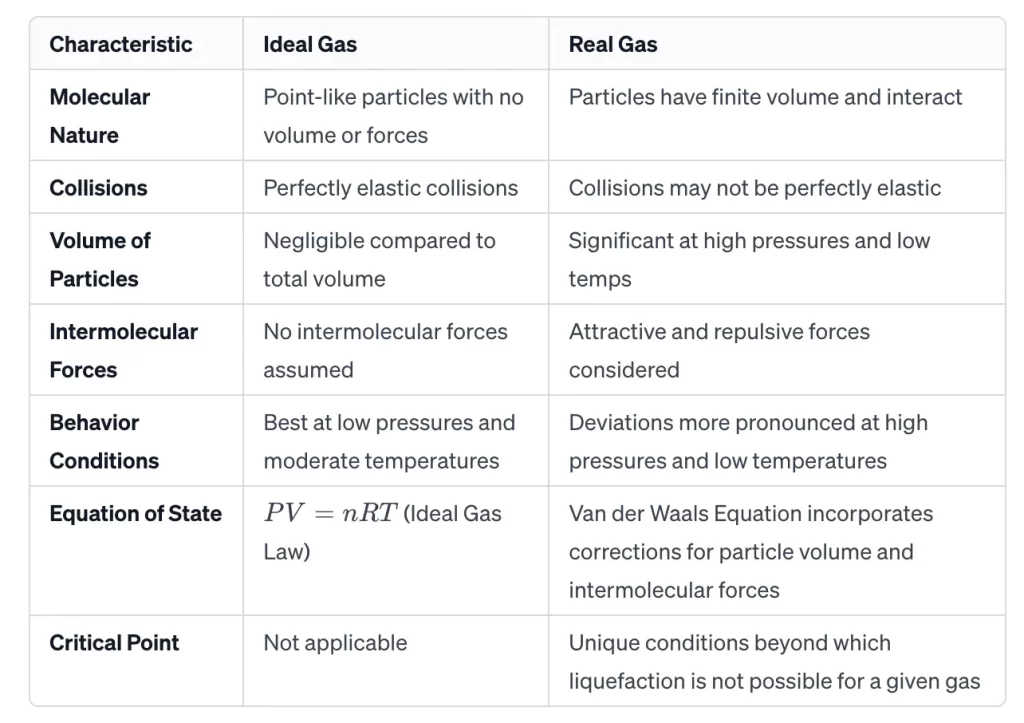
This chart provides a concise overview of the primary distinctions between ideal gases and real gases, summarising their assumptions, behaviours, and equations of state.




