In materials science, substances can be broadly classified into two main types based on their atomic structure: crystalline and amorphous materials. These two types differ significantly in how their atoms or molecules are arranged, which in turn affects their physical, mechanical, and thermal properties. In this blog, we will delve into the key differences between crystalline and amorphous materials, their properties, and examples of each type, to better understand how they are used in various industries.
What Are Crystalline Materials?
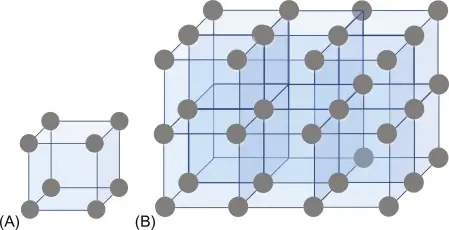
Crystalline materials are substances where atoms, ions, or molecules are arranged in a highly ordered and repeating pattern, extending in all three dimensions. This orderly atomic arrangement results in a material with a regular and predictable internal structure, which has significant implications for the material’s properties.
Key Features of Crystalline Materials:
- Ordered Structure: The defining feature of crystalline materials is their orderly, repeating atomic or molecular structure. The arrangement of atoms forms a crystal lattice, a regular geometric pattern that extends throughout the entire material.
- Sharp Melting Point: Crystalline materials tend to have a well-defined, sharp melting point. This is because the atoms are bonded in a highly ordered way, and the energy required to break these bonds is constant across the material.
- Anisotropy: Crystalline materials exhibit anisotropy, meaning their properties (like strength, conductivity, and refractive index) can vary depending on the direction in which they are measured within the material. This is due to the directional arrangement of the atoms in the lattice.
- Symmetry: Crystalline structures often exhibit symmetry, meaning the material can be divided into parts that are mirror images of each other along certain planes.
Examples of Crystalline Materials:
- Metals: Many metals, like iron, copper, and aluminium, are crystalline in nature. Their atoms are arranged in specific patterns such as face-cantered cubic (FCC), body-cantered cubic (BCC), or hexagonal close-packed (HCP) structures. The arrangement impacts properties like strength, ductility, and conductivity.
- Salt: Sodium chloride (NaCl), commonly known as table salt, is a well-known example of a crystalline material. The sodium and chloride ions arrange themselves in a repeating cubic structure, which gives salt its distinctive crystalline form.
- Quartz: Quartz (SiO₂) is a mineral with a well-defined crystalline structure. It is found in many forms, including crystals used in watches and other precision instruments due to its stability and predictability.
- Diamond: Diamond is one of the hardest known crystalline materials, with carbon atoms arranged in a tetrahedral lattice. This arrangement gives diamond its extraordinary hardness and high melting point.
What Are Amorphous Materials?
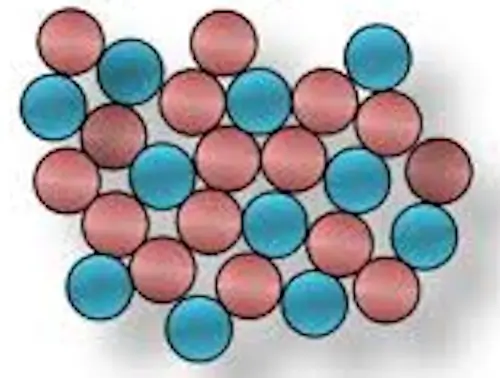
Amorphous materials, in contrast, lack the orderly atomic arrangement that characterizes crystalline substances. In these materials, the atoms or molecules are arranged in a disordered or random manner, similar to a liquid, but the material is in a solid state. As a result, amorphous materials exhibit different physical properties compared to crystalline ones.
Key Features of Amorphous Materials:
- Disordered Structure: Amorphous materials do not have a long-range order in their atomic or molecular structure. The atoms or molecules are randomly arranged, lacking the periodicity seen in crystalline materials.
- No Sharp Melting Point: Instead of a sharp melting point, amorphous materials tend to soften over a range of temperatures. This is because the disordered atomic structure does not have a uniform bond strength across the material.
- Isotropy: Amorphous materials are typically isotropic, meaning their properties are uniform in all directions. This is because the random arrangement of atoms does not favour any specific direction, unlike in crystalline materials.
- Glass Transition: Amorphous materials like glass exhibit a phenomenon known as the “glass transition,” where the material gradually transitions from a hard, brittle state to a more rubbery or flexible state when heated.
Examples of Amorphous Materials:
- Glass: Commonly used in windows, containers, and electronics, glass is a classic example of an amorphous material. It is primarily composed of silicon dioxide (SiO₂), which lacks long-range atomic order. The disordered arrangement contributes to glass’s transparency and brittleness.
- Rubber: Rubber, whether natural or synthetic, is an amorphous polymer. Its molecular chains are tangled in a random, disordered manner. This gives rubber its characteristic elasticity and flexibility.
- Plastics: Many synthetic plastics, such as polystyrene and polymethyl methacrylate (PMMA), are amorphous materials. The polymer chains in these materials are arranged in a disordered way, giving them their unique properties like transparency, flexibility, and ease of moulding.
- Gels: Many hydrogels and xerogels are amorphous in nature. These materials consist of a network of polymer chains that are randomly arranged, giving them properties such as high moisture retention and flexibility.
Crystalline vs Amorphous Materials: A Comparison
To better understand the differences between crystalline and amorphous materials, let’s summarize the key distinctions:
Property | Crystalline Materials | Amorphous Materials |
|---|---|---|
Atomic Structure | Ordered and repeating pattern | Disordered, random arrangement |
Melting Point | Sharp, well-defined melting point | Gradual softening over a range |
Symmetry | High symmetry (anisotropic) | No symmetry (isotropic) |
Mechanical Properties | Directional properties (anisotropy) | Uniform properties in all directions |
Examples | Metals, quartz, salt, diamond | Glass, rubber, plastics |
Properties | Hard, brittle, predictable | Flexible, transparent, moldable |
Applications of Crystalline and Amorphous Materials
Crystalline Materials:
- Electronics and Semiconductors: Crystalline silicon is widely used in the manufacture of microchips and solar cells due to its predictable electrical properties.
- Construction Materials: Crystalline materials like granite and marble are used in buildings and monuments because of their durability and aesthetic appeal.
- Jewellery: Crystals such as diamonds, sapphires, and emeralds are used in the creation of fine jewellery due to their hardness and beauty.
- Optical Applications: Crystalline substances like quartz are used in optical devices such as lenses and prisms because of their consistent refractive index.
Amorphous Materials:
- Glass: Used extensively in windows, containers, and optical lenses. Amorphous silica glass is also critical in fibre optics.
- Packaging: Plastics, which are often amorphous, are crucial in packaging materials due to their moldability and versatility.
- Medical Devices: Amorphous polymers are used in the medical field for making flexible and biocompatible implants and prosthetics.
- Coatings and Adhesives: Amorphous materials are often used as coatings or adhesives because their properties can be adjusted for specific applications, including flexibility and transparency.
Conclusion
In summary, crystalline and amorphous materials have fundamentally different atomic structures, which result in distinct physical, thermal, and mechanical properties. Crystalline materials are known for their ordered structure, sharp melting points, and directional properties, making them ideal for applications requiring precision and stability. In contrast, amorphous materials, with their disordered structure and isotropic properties, are valued for their flexibility, transparency, and ease of moulding, making them useful in everyday applications like glass and plastics.
Understanding the differences between these two types of materials helps in selecting the right material for specific uses, whether in electronics, construction, packaging, or medical devices.




