Niels Bohr’s atomic model, proposed in 1913, marked a revolutionary breakthrough in the understanding of atomic structure and laid the foundation for modern quantum physics. Building upon Rutherford’s nuclear model, Bohr introduced key concepts that provided a more accurate description of the behaviour of electrons within an atom.
Quantised Energy Levels:
Bohr introduced the concept of quantised energy levels for electrons. According to classical physics, an electron in orbit around the nucleus should continuously emit energy in the form of electromagnetic radiation, causing it to spiral into the nucleus. Bohr postulated that electrons could only occupy certain discrete orbits or energy levels, and these energy levels were stable.
Angular Momentum Quantisation:
Bohr quantised the angular momentum of electrons. He proposed that an electron’s angular momentum is quantised and is an integer multiple of ħ (Planck’s constant divided by 2π). This quantisation prevented the continuous loss of energy and the collapse of electrons into the nucleus, as classical physics predicted.
Mathematically, Bohr’s quantisation condition is expressed as:
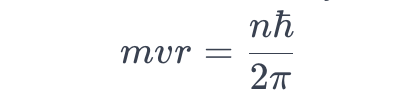
where
m is the mass of the electron,
v is its velocity,
r is the radius of the orbit,
n is the principal quantum number, and ℏ
ℏ is the reduced Planck’s constant.
Stationary Orbits:
Bohr proposed that electrons move in stable, circular orbits called “stationary orbits.” In these orbits, electrons do not emit radiation, and their energy remains constant. The centripetal force necessary to keep the electron in orbit is provided by the electrostatic attraction between the electron and the nucleus.
Energy Absorption and Emission:
Electrons can change orbits by absorbing or emitting discrete packets of energy, known as quanta. When an electron absorbs energy, it moves to a higher energy level (excited state). Conversely, when it emits energy, it transitions to a lower energy level (ground state). The frequency of the emitted or absorbed radiation is related to the energy difference between the initial and final orbits.
The energy of the emitted or absorbed radiation is given by the equation:
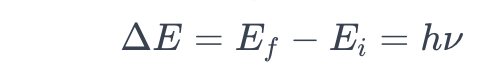
Application to Hydrogen Spectrum:
One of the significant successes of Bohr’s model was its ability to explain the spectral lines of hydrogen. The model accurately predicted the wavelengths of the lines in the hydrogen spectrum by considering the transitions of electrons between different energy levels.
While Bohr’s model successfully explained the hydrogen spectrum, it had limitations when applied to atoms with more than one electron. The model was eventually replaced by more sophisticated quantum mechanical models, such as the Schrödinger equation, which provided a more comprehensive and accurate description of atomic behaviour. Nonetheless, Bohr’s contributions laid the groundwork for the development of quantum mechanics and played a crucial role in the history of atomic theory.
Conclusion
Niels Bohr’s atomic model was instrumental in advancing our understanding of atomic structure and spectral phenomena. Despite being superseded by more comprehensive models, Bohr’s contributions to quantum theory remain foundational. His work laid the groundwork for the development of quantum mechanics, a field that continues to shape our understanding of the microscopic world.




