A UV-visible spectrophotometer is an essential instrument in analytical chemistry, widely used across various industries including pharmaceuticals, environmental science, food testing, and materials science. This powerful tool is used to measure the absorbance or transmission of ultraviolet (UV) and visible light by a sample, allowing researchers to analyze the chemical composition and concentration of substances. In this blog, we will delve into how the UV-visible spectrophotometer works, its components, applications, and important considerations when using the instrument.
What is a UV-Visible Spectrophotometer?
A UV-Visible spectrophotometer is an analytical device used to measure the amount of light absorbed by a sample in the ultraviolet (UV) and visible regions of the electromagnetic spectrum. The device typically covers the wavelength range of 190 to 1100 nanometers (nm), with the UV range spanning 190-400 nm and the visible range covering 400-700 nm.
By shining light through a sample, the spectrophotometer can identify how much light is absorbed at different wavelengths. The amount of absorption is directly related to the concentration of a compound in the sample, making UV-Vis spectroscopy a highly effective method for quantitative analysis.
Principle of UV-Visible Spectrophotometry
The basic principle behind a UV-visible spectrophotometer is Beer’s Law, which states that the absorbance (A) of a sample is directly proportional to the concentration (C) of the analyte in the sample, the path length (l) of the light through the sample, and the molar absorptivity (ε) of the analyte at a specific wavelength. Mathematically, it is expressed as:
A = ϵ ⋅ C ⋅ l
- A = Absorbance
- ε = Molar absorptivity (a constant that indicates how strongly the substance absorbs light at a particular wavelength)
- C = Concentration of the analyte
- l = Path length (the distance the light travels through the sample)
The UV-Visible spectrophotometer essentially shines light through the sample, measures how much light passes through (transmittance), and converts this into absorbance. The greater the absorbance at a particular wavelength, the more concentrated the substance is at that wavelength.
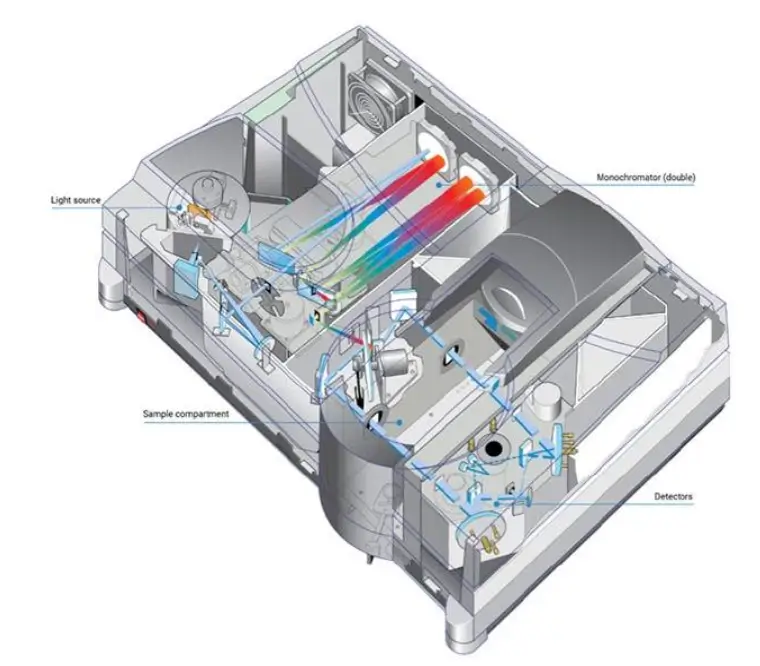
Components of a UV-Visible Spectrophotometer
A UV-Vis spectrophotometer consists of several key components that work together to measure light absorbance. These include:
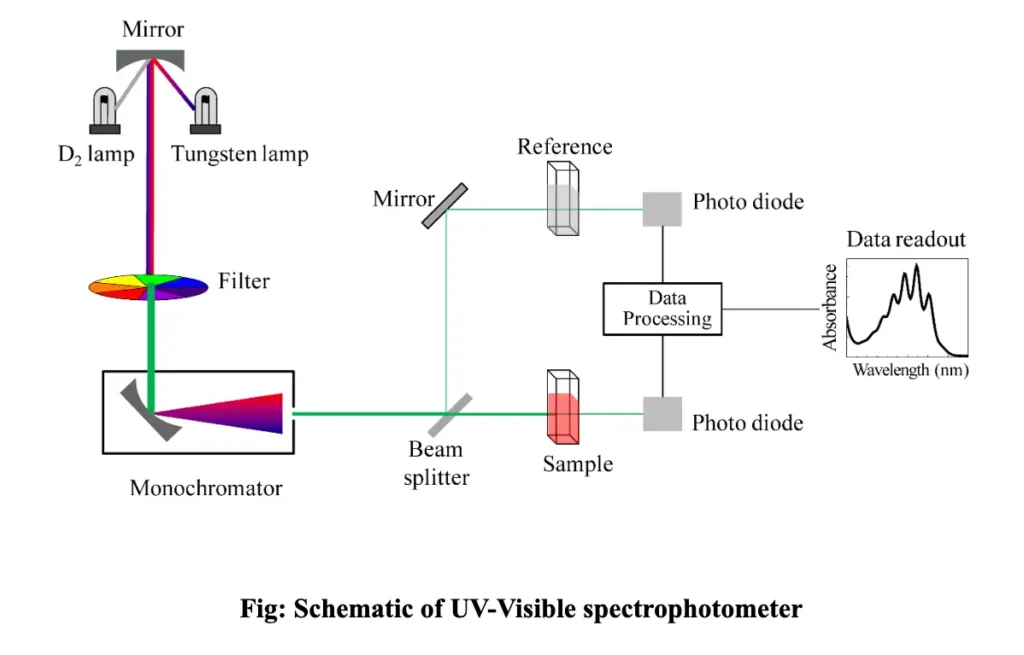
1. Light Source
The light source is responsible for generating the light that will be passed through the sample. UV-Vis spectrophotometers generally use two types of light sources:
- Deuterium Lamp: This lamp is used for the UV region (190-400 nm). It emits continuous light across this range, ideal for detecting UV absorbance.
- Tungsten Lamp: This lamp is used for the visible region (400-700 nm). It emits light in the visible spectrum and some infrared wavelengths.
In some high-end instruments, both light sources may be incorporated to ensure a broad wavelength coverage.
2. Monochromator
The monochromator is a device that selects specific wavelengths of light from the broader spectrum emitted by the light source. It works by using a diffraction grating or prism to disperse the light into its component wavelengths, allowing only one wavelength to pass through at a time. This is important because different molecules absorb light at different wavelengths, and precise control of wavelength is crucial for accurate analysis.
3. Sample Holder
The sample holder, or cuvette, holds the sample during analysis. Cuvettes are typically made of quartz, glass, or plastic, depending on the wavelength range being studied. For UV measurements (below 300 nm), quartz cuvettes are used because glass absorbs UV light, while plastic cuvettes are often used for the visible range.
4. Detector
The detector is used to measure the intensity of the light that passes through the sample. Photodetectors like photomultiplier tubes (PMTs) or photodiodes are used in UV-Vis spectrophotometers. They convert the light signal into an electrical signal that can be processed by the instrument.
5. Display and Data Processor
The final component is the display or data processor, which presents the results to the user. The data processor calculates the absorbance from the detected light intensity and may display the results as a spectrum, a numerical absorbance value, or a concentration value (if calibration data is available).
How a UV-Visible Spectrophotometer Works
Step 1: Light Generation
The process begins with the light source emitting light across a range of wavelengths, from UV to visible.
Step 2: Wavelength Selection
The monochromator selects the specific wavelength of light to pass through the sample, typically adjusted by the user based on the absorbance characteristics of the substance being analysed.
Step 3: Sample Interaction
The selected light passes through the sample. Some of the light is absorbed by the sample, while the rest transmits through. The amount of light absorbed depends on the chemical composition of the sample.
Step 4: Detection
The detector measures the intensity of the transmitted light, comparing it with the intensity of light before it passed through the sample.
Step 5: Data Analysis
The spectrophotometer processes the data and calculates the absorbance. The instrument may also generate a spectrum, which is a plot of absorbance versus wavelength. By analysing this spectrum, the user can identify the presence and concentration of specific compounds.
Applications of UV-Visible Spectrophotometry
UV-visible spectrophotometry has a wide range of applications across many industries. Some common uses include:
1. Pharmaceutical Industry
- Quantitative analysis: Determining the concentration of active pharmaceutical ingredients (APIs) in formulations.
- Quality control: Testing raw materials and final products for consistency and purity.
- Stability studies: Monitoring the degradation of drugs over time.
2. Environmental Science
- Water quality testing: Measuring pollutants and contaminants such as nitrates, phosphates, and heavy metals in water samples.
- Air quality monitoring: Analysing gases and particulate matter in the air.
3. Food and Beverage Industry
- Food analysis: Measuring the concentration of additives, preservatives, and pigments in food and beverages.
- Quality control: Ensuring consistency in product colour and concentration.
4. Biochemical Research
- Protein and nucleic acid quantification: Using absorbance at specific wavelengths (e.g., 260 nm for nucleic acids and 280 nm for proteins) to quantify concentrations.
- Enzyme activity assays: Measuring the absorption of light at particular wavelengths in reaction mixtures to evaluate enzyme kinetics.
5. Materials Science
- Polymer characterization: Analysing the optical properties of polymers and other materials.
- Nanomaterials: Measuring the absorption spectra of nanoparticles and nanomaterials for research purposes.
Key Considerations in Using a UV-Visible Spectrophotometer
When using a UV-visible spectrophotometer, there are several important considerations to ensure accurate results:
1. Sample Preparation
Proper sample preparation is essential for obtaining accurate results. The sample must be free from contaminants, and the concentration should be within the linear range of the spectrophotometer. Samples that are too concentrated may require dilution.
2. Calibration
The instrument should be regularly calibrated using known standards to ensure reliable and accurate measurements. Calibration curves can help quantify the concentration of unknown samples based on their absorbance at specific wavelengths.
3. Path Length
The path length (the distance light travels through the sample) affects absorbance. Standard path lengths are usually 1 cm, but some samples may require longer or shorter path lengths. It is essential to ensure consistency in path length across samples.
4. Solvent Selection
The choice of solvent can impact the absorbance spectrum of a sample. Solvents should be selected carefully to ensure that they do not absorb significantly at the wavelengths of interest.
5. Instrument Maintenance
Regular maintenance and cleaning of the instrument components (especially the cuvettes and optical components) are necessary to prevent contamination and ensure the instrument operates at its best.
Conclusion
A UV-visible spectrophotometer is a versatile and indispensable tool in modern analytical laboratories. By providing precise measurements of light absorption, it enables researchers to determine the concentration of substances, analyse chemical reactions, and monitor product quality across diverse industries. Understanding how this instrument works and how to use it effectively can enhance its potential to provide accurate, reliable results. Whether you are analysing pharmaceutical compounds, testing water quality, or conducting biochemical research, the UV-visible spectrophotometer plays a crucial role in delivering the insights necessary for success.




