Nucleons in the Nucleus Definition
Nucleons are the subatomic particles that make up the atomic nucleus. The nucleus of an atom consists of two types of nucleons: protons and neutrons. Protons are positively charged particles, while neutrons carry no charge (neutral). Together, these nucleons are held in the nucleus by the strong nuclear force, which is one of the four fundamental forces of nature and acts over very short ranges.
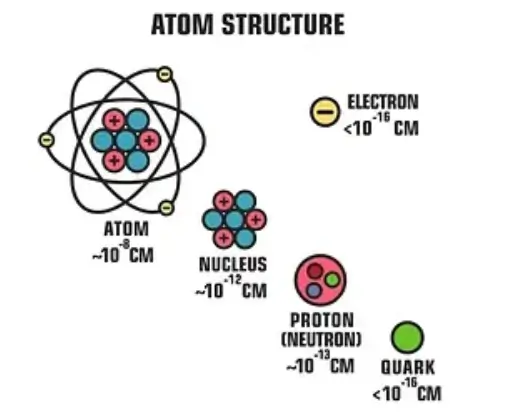
Discovery
The discovery of nucleons was a pivotal moment in the development of modern atomic theory and nuclear physics.
Discovery of the Proton
The proton was discovered by Ernest Rutherford in 1917 during his experiments on the scattering of alpha particles by nitrogen gas. Rutherford observed that when alpha particles (helium nuclei) collided with nitrogen nuclei, hydrogen nuclei (protons) were ejected. This led him to propose the existence of the proton as a fundamental constituent of the nucleus. Rutherford’s work built on his earlier gold foil experiment, which had already revealed the presence of a dense, positively charged nucleus at the centre of the atom.
Discovery of the Neutron
The neutron was discovered by James Chadwick in 1932. Prior to its discovery, the existence of a neutral particle in the nucleus was hypothesised to explain the disparity between atomic number (number of protons) and atomic mass. Chadwick conducted experiments bombarding beryllium with alpha particles, which produced a new type of radiation. This radiation was able to penetrate substances more deeply than protons and was not deflected by electric or magnetic fields, indicating it was neutral. Through careful experimentation, Chadwick was able to demonstrate that this radiation consisted of particles with a mass similar to that of protons, leading to the identification of the neutron.
Types of Nucleons
There are two main types of nucleons:
Protons
Protons are positively charged particles found in the nucleus of an atom. Each proton has a charge of +1e (where e is the elementary charge) and a mass of approximately 1 atomic mass unit (amu), or 1.6726 × 10^-27 kilograms. The number of protons in the nucleus of an atom determines the element’s atomic number and thus its identity. For instance, hydrogen has one proton, carbon has six, and uranium has ninety-two.
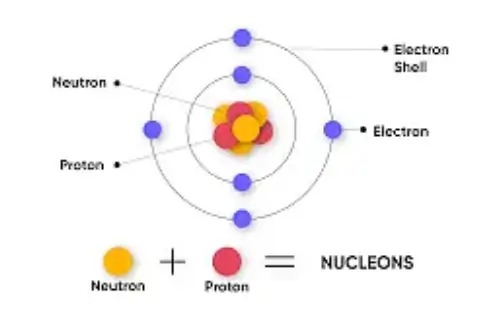
The proton is composed of three quarks: two up quarks and one down quark, held together by the strong force mediated by gluons. This internal structure makes the proton a baryon, a type of hadron.
Neutrons
Neutrons are neutral particles that reside in the nucleus alongside protons. They have no electric charge and a mass slightly greater than that of protons, approximately 1.6750 × 10^-27 kilograms. Neutrons play a critical role in the stability of the nucleus, providing an attractive force that helps to offset the electrostatic repulsion between positively charged protons.
Neutrons, like protons, are also baryons, consisting of three quarks: one up quark and two down quarks. The presence of neutrons affects the isotopic characteristics of an element, as atoms of the same element can have varying numbers of neutrons, leading to different isotopes.
Role of Nucleons in the Nucleus
Nucleons are bound together in the nucleus by the strong nuclear force, which is much stronger than the electromagnetic force but acts only over very short distances (about 1 femtometer). This force ensures the stability of the nucleus, allowing it to overcome the repulsive electromagnetic force between positively charged protons.
The number of neutrons relative to protons in the nucleus determines the stability of the nucleus. A proper balance is necessary; too many or too few neutrons can lead to instability and radioactive decay. For light elements, stable nuclei often have nearly equal numbers of protons and neutrons, while heavier elements typically require more neutrons than protons to remain stable.
Importance in Nuclear Reactions
Nucleons are crucial in nuclear reactions, such as fission and fusion. In fission, heavy nuclei split into smaller nuclei, releasing energy and more neutrons, which can trigger further fission reactions (a chain reaction). In fusion, light nuclei combine to form a heavier nucleus, releasing energy. These processes are the foundation of nuclear power and have applications in both energy production and weapons.
Conclusion
Nucleons, comprising protons and neutrons, are fundamental components of atomic nuclei. Their discovery was key to advancing our understanding of atomic structure and nuclear physics. By understanding nucleons and their interactions, scientists have been able to unlock the power of the nucleus, leading to both beneficial technologies and important insights into the fundamental nature of matter.




